Marielle Renae De Baun, OMS 2026; Glynnis Page, OMS 2026
Rocky Vista University College of Osteopathic Medicine
DOI: 10.70709/r2fykae2uu
Abstract
Background
The use of glucagon-like peptide-1 receptor agonists (GLP-1s) has surged in response to the growing prevalence of type 2 diabetes mellitus and obesity—two conditions that are known to elevate the risk of perioperative complications in orthopedic surgery. Despite the widespread adoption of GLP-1s, limited research exists examining their implications within the field of orthopedics. This review aims to evaluate the current evidence on GLP-1s as it relates to bone metabolism, wound healing, and surgical outcomes, with the goal of informing clinical decision-making and guiding future research in orthopedic surgical care.
Methods
A comprehensive literature review of PubMed, Embase, and Elsevier databases identified 17 relevant studies published between 2013 and 2024 that investigated the role of GLP-1 receptor agonists in bone metabolism, wound healing, and orthopedic surgery. Studies published before 2013, non-English language articles, and case reports were excluded. Given the heterogeneity of study designs and outcomes, results were synthesized using a narrative approach.
Results
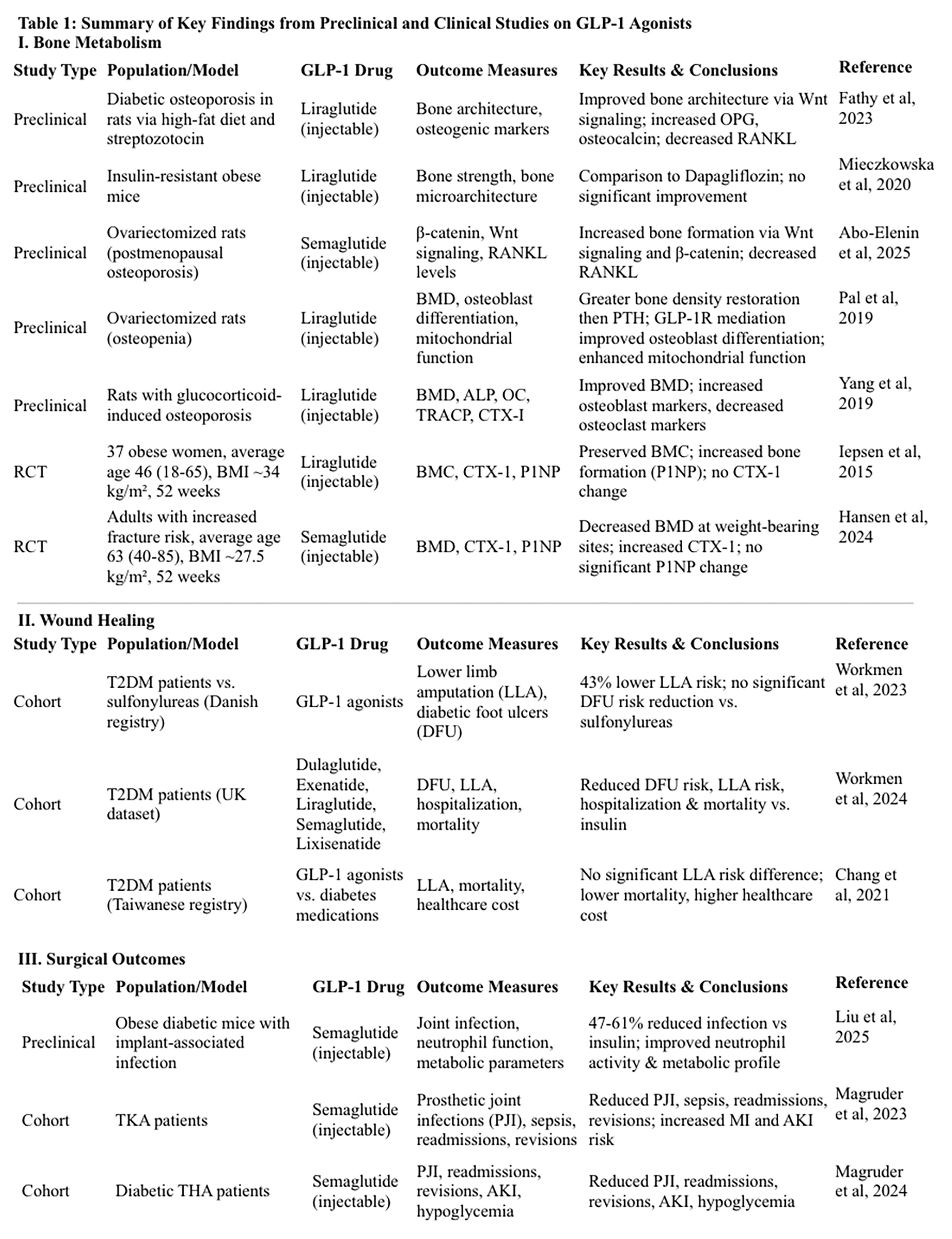
Bone metabolism: Five preclinical studies found GLP-1 agonists enhanced osteoblastic differentiation and increased bone formation in osteoporotic and diabetic models. Four literature reviews concluded GLP-1s increased bone formation, three of which found them superior to other diabetes medications. Two randomized control clinical trials reported conflicting effects on bone mineral content (BMC) and bone mineral density (BMD): one trial demonstrated preservation of BMC with GLP-1 use, while the other found no significant difference in BMC or BMD between treatment and control groups.
Wound healing: Three studies associated GLP-1s with reduced all-cause mortality. Two studies reported a significant reduction in lower limb amputations (LLA) and diabetic foot ulcers, while one study found no significant difference in LLA compared to other diabetic therapies.
Surgical outcomes: A preclinical model found a 47% reduction in periprosthetic joint infections with GLP-s compared to insulin and a 61% reduction compared to untreated diabetic controls. Additionally, reactive oxygen species production increased by 22% and neutrophil migration by 30%, indicating enhanced immune response. Two cohort studies further supported these findings, showing GLP-1 use correlated with reduced prosthetic joint infections, fewer 90-day readmission rates, and reduced 2-year revisions in total knee and hip arthroplasty patients.
Conclusion
GLP-1s directly influence bone remodeling through receptor expression on osteocytes and bone mesenchymal stem cells. Emerging evidence suggests they may exert anabolic effects on bone, mitigate skeletal damage associated with obesity and diabetes, promote wound healing, and reduce perioperative complications. Despite these promising findings, long-term clinical data remains limited. Further research is essential to clarify their role and optimize their integration into perioperative management protocols for orthopedic patients.
Keywords: GLP-1 receptor agonists; orthopedic surgery; GLP-1 agonist signaling; bone metabolism; perioperative complications; diabetes
Introduction
The United States faces a profound and worsening obesity crisis, with recent CDC data estimating that over 40% of U.S. adults are obese and approximately 1 in 11 suffer from severe obesity. (1) This growing population faces elevated risks of developing metabolic complications, notably type 2 diabetes, and is increasingly in need of effective and sustainable therapeutic strategies.
Glucagon-like peptide-1 (GLP-1) receptor agonists have emerged as a novel and effective solution, having initially been approved and marketed for glycemic control in type 2 diabetes. (2) A rapid surge in popularity has occurred, with prescription volumes increasing by 300% between 2020 and 2022 alone. (3) Notably, only 53.8% of those prescribed a GLP-1 agonist in 2022 had a history of type 2 diabetes, highlighting a shifting clinical paradigm in which these agents are now widely used for obesity management and related metabolic disorders. (3,4) Once considered primarily tools for glycemic control, GLP-1 receptor agonists have now become first-line medications for addressing obesity and related complications. (4)
Changes in prescribing trends have been facilitated by a growing number of available GLP-1 receptor agonists, with currently ten agents approved for clinical use. Among the most recognized is semaglutide, available in injectable form as Ozempic for type 2 diabetes and Wegovy for weight loss, as well as an oral formulation, Rybelsus. Liraglutide also has dual branding, having a diabetic formulation marketed as Victoza for diabetes and Saxenda for weight management. Additional GLP-1 agonists include exenatide (brand name Byetta), lixisenatide (Adlyxin), and dulaglutide (Trulicity). Tirzepatide (Mounjaro) is currently the only approved dual agonist of both GLP-1 and GIP receptors. (5) As these agents become increasingly integrated into clinical practice, their effects on weight, metabolic regulation, and systemic inflammation carry important implications for orthopedic surgery. Given the challenges obesity poses in this field—including increased perioperative risk, altered musculoskeletal biomechanics, and poorer long-term outcomes—GLP-1 agonists may play a valuable role in optimizing orthopedic surgical care.
Against this backdrop of rising obesity and new pharmacologic strategies, it is critical to note that the majority of patients undergoing orthopedic surgery, particularly joint arthroplasty, meet criteria for obesity (BMI 30–39.9) or morbid obesity (BMI ≥40). (6) There are analyses indicating that more than half of all arthroplasty patients are obese, with one large retrospective cohort of 8.3 million individuals reporting that 64% of primary total knee arthroplasty (TKA) patients and 49% of primary total hip arthroplasty (THA) patients are obese. (6,7) Both estimates are notably higher than the adult obesity projection of approximately 40%. (1) Given that osteoarthritis (OA) is the leading indication for arthroplasty and obesity is the most significant modifiable risk factor for OA development, it is unsurprising that a substantial proportion of arthroplasty patients are affected by obesity. (8) Excess body weight increases the mechanical load on weight-bearing joints, accelerating cartilage wear and joint degeneration, and contributing to a pro-inflammatory state that further exacerbates joint damage. (8)
Obesity and bone have both a mechanical and metabolic relationship. Due to mechanical loading, increased body weight is positively correlated with bone mineral density (BMD) and cortical bone formation. (9) Obesity has also been demonstrated to be protective against osteoporosis due to estrogen production from adipose tissue. (10) However, the positive effects of obesity on bone formation are nullified by the simultaneous induction of functional osteopenia, in a phenomenon known as the obesity paradox. (10) Several mechanisms are involved, creating a complex interplay that weakens bone and increases propensity for injury. Central obesity increases risk of falling through postural instability from an anterior shift of the center of gravity. (9) Comorbidities of obesity and diabetes may also increase predisposition for falls, including peripheral neuropathy, obstructive sleep apnea, osteoarthritis, and femoral avascular necrosis. (9, 11)
At the metabolic level, the signaling cascades that regulate bone metabolism offer some explanation for the obesity paradox. Homeostatic bone requires a balance of bone formation and resorption. Osteoblasts are responsible for bone formation, with capacity for terminal differentiation into osteocytes, the most abundant bone cell and regulator of matrix mineralization. (9) Bone resorption is accomplished through the enzymatic activity of osteoclasts. Osteoblasts share a common progenitor with adipose tissue, and in obese metabolic environments, bone mesenchymal stem cells (BMSCs) preferentially differentiate into bone marrow adipose tissue (BMAT). (9) BMAT acts as a structural and metabolic component, being insulin sensitive with endocrine and osteogenic remodeling activity. Replacement of osteogenic cells with BMAT decreases bone quality and contributes to an overall poor metabolic environment. (9)
Risk of perioperative complications increase proportionally to BMI. Soft tissue and altered biomechanics in patients with a high BMI can make surgical exposure and component placement more challenging, leading to prolonged operative times. (12) Additionally, higher BMI is strongly correlated with a greater likelihood of surgical site infections, wound dehiscence, venous thromboembolism, and cardiopulmonary events. (12) Moreover, the frequent coexistence of type 2 diabetes mellitus compounds these risks by compromising wound healing and weakening the immune response, thereby magnifying the danger of postoperative infections. (12) These challenges lengthen hospital stays, increase the need for readmissions or revision procedures, and impair long-term functional outcomes. (12) The growing number of orthopedic patients with obesity thus underscores an urgent need for interventions that target both weight reduction and metabolic optimization, offering the potential to improve overall surgical safety and long-term musculoskeletal health. Addressing obesity through weight management strategies, including pharmacologic ones like GLP-1 receptor agonists, is a key component of reducing all operative risks, improving surgical outcomes, and enhancing the long-term success of orthopedic interventions.
Beyond their well-established metabolic benefits, growing evidence suggests that GLP-1 receptor agonists exert both direct and indirect effects on bone health. GLP-1 receptors have been identified in bone mesenchymal stem cells, indicating a broader physiological role for these incretin hormones. (12, 13) By improving glycemic control, enhancing insulin sensitivity, and reducing adiposity, GLP-1 therapies may decrease bone marrow adipose tissue (BMAT) formation and mitigate the harmful effects of hyperglycemia on bone quality. (13) Additionally, GLP-1 signaling may help suppress pro-inflammatory pathways, reducing the chronic low-grade inflammation associated with both obesity and bone loss. (13)
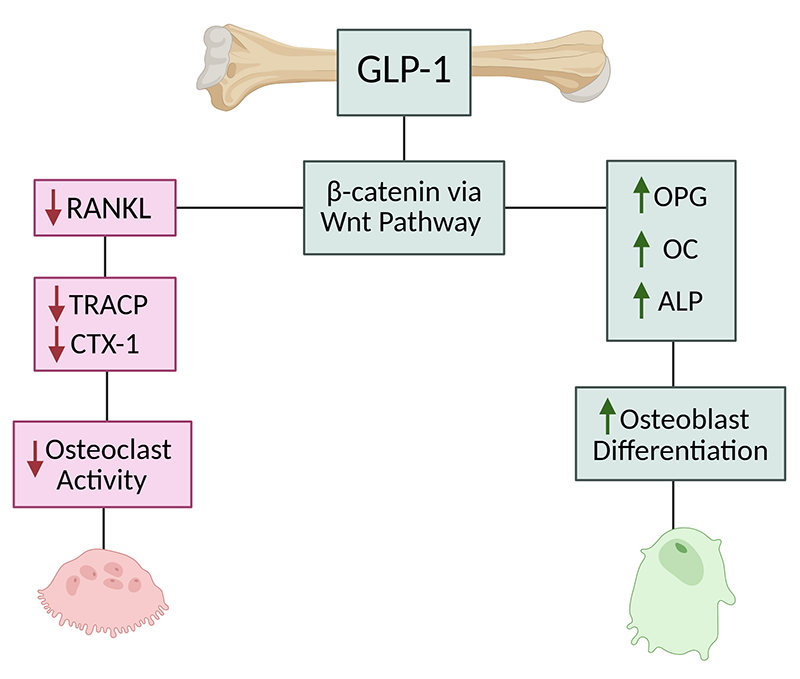
A key mechanism through which GLP-1 receptor agonists may influence bone metabolism is the osteoprotegerin (OPG)/RANKL/RANK pathway, which regulates osteoclast differentiation and bone resorption. (13) RANKL promotes osteoclast formation and activity, whereas OPG acts as a decoy receptor, inhibiting this process and protecting against bone loss. (13) Disruption of this balance—such as elevated RANKL or reduced OPG—is linked to increased bone resorption and delayed fracture healing, particularly in diabetes. (13) Interestingly, elevated OPG levels observed in patients with type 1 diabetes may reflect a compensatory response to increased bone turnover. (13) Preclinical studies have demonstrated that GLP-1 receptor agonists like exenatide can enhance bone mass in diabetic and insulin-resistant rat models by upregulating OPG expression and improving the OPG/RANKL ratio. (13) These findings suggest a promising osteogenic role for GLP-1 therapies, especially in metabolic disease contexts. (13)
Thus, as GLP-1 receptor agonists promote weight loss and enhance metabolic function, they could alter the secretion of osteogenic cytokines and adipokines to promote bone formation and resorption. (14) These emerging connections between GLP-1 signaling and skeletal physiology present a biologic rationale for how GLP-1 receptor agonists have potential to not only assist in weight management, but also favorably influence bone health and remodeling.
This review examines GLP-1 receptor agonists within the orthopedic context, exploring how their metabolic and anti-inflammatory effects may influence patient care, surgical outcomes, and postoperative recovery.
Methods
This literature review was conducted to investigate the impact of GLP-1 agonists on bone health in orthopedic patients. A systematic search was employed using medical research databases including PubMed, Embase, and Elsevier. The search terms used included “GLP-1 agonists,” “Glucagon-Like Peptide-1 Receptor Agonists,” “semaglutide,” “Ozempic,” “Wegovy,” “liraglutide,” “bone health,” “orthopedic surgery,” “arthroplasty,” “post-operative complications,” “complications,” “bone health,” “bone strength,” “osteogenesis,” and “bone metabolism.”
To ensure the relevance and quality of the studies, we established specific inclusion and exclusion criteria. We included peer-reviewed articles written in English that investigated the impact of GLP-1 RAs on bone health, bone metabolism, or bone remodeling in human subjects or relevant animal models. Studies had to report outcomes related to bone mineral density, fracture risk, bone healing, or postoperative complications affecting bone health. We excluded publications prior to 2013, non-English articles, case reports, conference abstracts, editorials, and studies lacking sufficient bone-related data.
Studies were screened in two stages—title and abstract review, followed by full-text review—conducted independently by two reviewers. Data on study design, sample size, interventions, and outcomes were extracted using a standardized form. Quality assessment was performed using the Cochrane risk-of-bias tool. A narrative synthesis approach was used to summarize findings across studies due to variability in study designs and outcome measures.
The selection process involved an initial screening of titles and abstracts by two independent reviewers to identify studies that met our criteria. Full-text articles were then obtained for those that appeared relevant or where eligibility was uncertain. Any discrepancies between the reviewers were resolved through discussion to reach a consensus.
For each study included in the review, we extracted detailed information on the study design, sample size, participant characteristics, type and dosage of GLP-1 RA used, and the key outcomes measured. Particular attention was given to results concerning changes in bone mineral density, levels of bone turnover markers, fracture incidence, and bone healing processes.
Given the diversity of study designs and outcome measures, we synthesized the findings narratively rather than quantitatively. This approach allowed us to explore broad themes such as the effects of GLP-1 RAs on osteoblast and osteoclast activity, bone formation and resorption, and the overall impact on bone integrity and healing. We also considered potential biological mechanisms underlying these effects, integrating insights from molecular and physiological studies.
Throughout this review, we remained cognizant of potential limitations, such as publication bias and variability in study quality. While we assessed the methodological rigor of each study using appropriate quality assessment tools, we included all relevant studies to provide a comprehensive overview of the current literature. Our goal was to elucidate the complex relationship between GLP-1 receptor agonists and bone health, particularly in the context of orthopedic surgery, to inform clinical practice and identify areas for future research.
Results
A total of 17 studies were analyzed that addressed the impact of GLP-1 receptor agonists on bone health, bone and joint infections, and postoperative outcomes. The studies included various experimental designs, such as randomized controlled trials, retrospective cohort studies, and preclinical animal models. The major findings from these studies are summarized below.
GLP-1 Effects on Bone Metabolism
Pre-Clinical Studies
Several animal models were conducted to demonstrate molecular signaling pathways of GLP-1 agonists. These studies explored how GLP-1 agonists influence bone metabolism in an osteoporotic state, including effects on bone turnover markers, BMD, and fracture risk. Osteoporosis was experimentally induced using a high-fat diet with streptozotocin in two studies, ovariectomy in two studies, and dexamethasone administration in one study.
Liraglutide was found to improve bone microarchitecture and reverse bone loss in rats with diabetic osteoporosis. (15) After inducing diabetic osteoporosis through a high fat diet and streptozotocin, light and scanning electron microscopy revealed evidence of bone deterioration via the upregulation of nuclear factor kappa-B ligand (RANKL) and downregulation of pro-osteogenic markers OPG and osteocalcin. Upon treatment with liraglutide, the effects of diabetic osteoporosis were reversed. Bone formation occurred through GLP-1 regulation of the Wnt signaling pathway, resulting in a reduction of RANKL expression and increased levels of OPG and osteocalcin. (15) In a separate animal study by Mieczkowska et al., a comparison of liraglutide to dapagliflozin on bone strength and microarchitecture in insulin-resistant and obese mice found no indication of improved bone strength or microarchitecture by either substance. (16)
Abo-Elenin et al. studied the effects of semaglutide in ovariectomized rats as a model for postmenopausal osteoporosis. (17) Semaglutide was found to enhance bone formation through the upregulation of osteogenic pathways- specifically β-catenin via the Wnt signaling pathway. (17) Likewise, there was a reduction in the production of RANKL, thereby decreasing osteoclast mediated bone resorption. (17) Another study using ovariectomized rats as model for osteopenia found that liraglutide was able to fully restore bone mass and strength. (18) The effects of liraglutide were compared to parathyroid hormone (PTH), with greater bone mineral density normalization by liraglutide than PTH. (18) The study further revealed that the effects of liraglutide on osteoblast differentiation and mitochondrial function were mediated through the GLP-1 receptor (GLP1R), as blocking GLP1R or AMP-activated protein kinase (AMPK) inhibited these outcomes. (18) Liraglutide also enhanced mitochondrial number and respiratory function, which contributed to improved osteoblast differentiation. (18)
Yang et al. used dexamethasone to create a state of glucocorticoid-induced osteoporosis. (19) Liraglutide was found to improve bone mineral density and mitigate the detrimental effects of glucocorticoids on bone metabolism. (19) Bone formation indicators alkaline phosphatase (ALP) and osteocalcin (OC) increased with the addition of liraglutide, meanwhile bone resorption indicators tartrate-resistant acid phosphatase (TRACP) and cross-linked carboxy-terminal telopeptide of type I collagen (CTX-I) were reduced. (19)
Clinical Studies
Two randomized control trials studied the effect of GLP-1 receptor agonists on BMD, bone mineral content (BMC), and osteogenic markers. The results were varied on whether GLP-1 agonists have the potential to preserve BMD and BMC. In a trial by Iepsen et al., 37 women with an average age of 46 (Standard Deviation: 2 years, range 18-65 years) and BMI of 34 (SD: 0.5 kg/m2) achieved a 12% weight loss after 8 weeks of low-calorie diet modifications. (20) Baseline measurements of total, pelvic, and arm-leg BMC, CTX-1 and Procollagen Type I N-terminal propeptide (P1NP) were obtained before participants were assigned to the study or control group. (20) Each group included 7 postmenopausal women, with 18 total women assigned to the liraglutide group and 19 to the control group. (20) To isolate the effects of liraglutide on bone metabolism independent of weight loss, both groups were instructed to maintain their weight. (20) Liraglutide was dosed at 1.2mg/d, and low-calorie substitutions were permitted for both the control and study group as needed for weight maintenance. (20) After 52 weeks, participants in both groups maintained their weight loss of 12% and BMC, CTX-1, and P1NP were measured again. (20) The control group experienced four times more BMC loss compared to the liraglutide group, with an approximate difference of 27g (95% CI: 5-48, p=.01). (20) The liraglutide group also showed a 16% increase in the bone formation marker P1NP (7g/L, SD: 3.3) as compared to the control group with a 2% increase (1g/L, SD: 4, p=<.05). (20) Bone resorption marker CTX-1 remained unchanged in both groups, suggesting that the primary mechanism of liraglutide’s bone-preserving effects is enhanced bone formation rather than decreased resorption. (20)
In separate study, Hansen et al. evaluated the effect of semaglutide in a 52-week randomized, double-blinded, placebo-controlled trial involving adults with increased fracture risk. (21) Participants had an average age of 63 (SD: 5.5, range 40-85 years) and an average BMI of 27.5 (SD: 4.5), consisting of 55 postmenopausal women and 9 men. (21) Increased fracture risk was determined by a T score of <-1.0 and/or a low energy fracture within the past three years. (21) Baseline measurements of P1NP, BMD, and CTX-1 were recorded, and participants were randomized into the semaglutide group to receive 1.0 mg or the placebo group. (21) During the trial, 9 participants in the semaglutide group required dose reductions due to adverse effects. (21) After 52 weeks, there was no significant difference in P1NP between semaglutide and placebo groups, with an estimated difference of 3.8 μg/L (95% CI: −5.6-13.3, p = 0.418). The semaglutide group had increased CTX-1, with an estimated difference of 166.4 μg/L (p = 0.021), and reduced BMD in the lumbar spine (ETD: -0.018 g/cm²; p = 0.007), total hip (ETD: -0.020 g/cm²; p = 0.001), tibia (ETD: -3.6 mg/cm³; p = 0.003), and reduced tibial cortical thickness (ETD: -1.8%; p = 0.012). (21) Bone loss was most pronounced in weight-bearing regions, with no significant effects on non-weight-bearing sites such as the radius. (21) These findings suggest that bone mass reductions and resorption increase are associated with weight loss rather than direct drug effects. (21)
Literature Reviews
Four literature reviews were included: three compared GLP-1 receptor agonists to other hypoglycemic agents, and one focused specifically on their effects on bone health. A review by Palermo et al. compared GLP-1 agonists to other hypoglycemic agents, including metformin, sulfonylureas, thiazolidinediones, meglitinides, gliptins, and SGLT2 inhibitors. (22) They identified that GLP-1s were superior to all other medications at increasing bone formation in animal in vitro and in vivo studies, as well as in human in vitro studies. (22) Wikarek et al. came to similar conclusions, finding that GLP-1 agonists were able to increase BMD and reduce fracture risk compared to other diabetes drugs. (23) Antonopoulou et al. expounded on the obesity paradox, suggesting increased BMD is offset by poor bone quality due to advanced glycation end-products (AGEs) accumulation, impaired osteoblast function, and uncoupled bone turnover favoring resorption. (24) Preclinical animal models demonstrated that GLP-1 receptor agonists may help protect against fractures and reduce advanced glycation end-product (AGE) accumulation in diabetic subjects. (24) Additionally, a narrative review by Herrou et al. supported the anabolic effects of GLP-1s on bone formation, particularly in the context of weight loss; however, the review noted that most included studies focused on patients with diabetes rather than those with obesity. (25)
GLP-1 Effects on Wound Healing
A Werkmen et al. (2023) study examined the impact of GLP-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors (SGLT2-Is) on lower limb amputation (LLA) risk using Danish National Health Service registry data from 2013 to 2018. (26) This retrospective cohort study included 74,475 patients with type 2 diabetes taking either an SGLT2-I, GLP-1, or sulfonylurea, with sulfonylureas serving as the reference group. (26) GLP-1 use was associated with a 43% lower risk of LLA compared to sulfonylureas, with an adjusted hazard ratio (HR) of 0.57 (95% CI: 0.39–0.84). (26) The greatest risk reduction occurred in long-term users (HR: 0.44, 95% CI: 0.27–0.72). (26) No significant differences in diabetic foot ulcer (DFU) risk were observed for GLP-1 use (HR: 1.08; 95% CI: 0.87–1.35) compared to sulfonylureas. (26) Stratification by cumulative dose showed enhanced protective effects at higher doses. (26) GLP-1 agonists also demonstrated consistent reductions in lower limb amputation risk across subgroups, including those with peripheral arterial disease (PAD) and patients on antihypertensive therapy. (26)
A follow up study by Werkman et al. (2024) specifically investigated the impact of GLP-1 agonists on diabetic foot ulcers and related complications, including lower limb amputation, DFU-related hospitalizations, and mortality. (27) Using data from the Clinical Practice Research Datalink Aurum, a propensity score-matched retrospective cohort study involving 25,422 participants with type 2 diabetes was conducted to compare the outcomes of patients who initiated GLP-1s vs. insulin and DPP4-Is vs. sulfonylureas. (27) Specifically, dulaglutide, exenatide, liraglutide, semaglutide, and lixisenatide were compared. (27) The study demonstrated that GLP-1 use was associated with a significantly lower risk of DFU compared to insulin (HR: 0.44, 95% CI: 0.32–0.60) for short-term use (≤400 days) and long-term use (HR: 0.74, 95% CI: 0.60–0.92). (27) Additionally, GLP-1 agonists were linked to lower hospitalization rates, mortality, and a reduced risk of lower limb amputation (HR: 0.83, 95% CI: 0.67–1.03). (27) Similar but less pronounced benefits were observed with DPP4-I therapy compared to sulfonylureas (HR 0.88, 95% CI: 0.79–0.97 for DFU risk reduction). (27) The benefits of GLP-1 agonists were observed across various subgroups, including males and females, individuals younger than 70 years, and patients with varying diabetes durations. (27)
Chang et al. conducted a nationwide retrospective cohort study using the Taiwan National Health Insurance Research Database from 2015 to 2017 to evaluate the risks of lower limb amputation, all-cause mortality, and healthcare costs associated with different antidiabetic therapies. (28) The study included 1,222,436 patients with type 2 diabetes who were newly prescribed SGLT-2 inhibitors, GLP-1 agonists, DPP4-Is, or older antidiabetic agents (metformin, sulfonylureas, alpha glucosidase inhibitors, thiazolidinediones), with patients categorized based on their first recorded prescription. (28) There were no statistically significant differences in amputation risk between GLP-1s, SGLT-2 inhibitors, DPP4-Is, and all other agents. (28) The crude incidence rates of amputation were 6.1 per 10,000 person-years for GLP-1s, 1.5 for SGLT-2 inhibitors, 4.6 for DPP4-Is, and 1.9 for other agents. (28) The adjusted hazard ratio for amputation did not significantly differ across drug classes. (28) GLP-1 users had a lower all-cause mortality rate (18.3 per 10,000 person-years) compared to DPP4-Is (69.9 per 10,000 person-years) and older agents (52.5 per 10,000 person-years). (28) Healthcare costs varied across treatment groups, with GLP-1s having the highest average daily costs (NTD$364) compared to SGLT-2 inhibitors (NTD$235), DPP4-Is (NTD$253), and older agents (NTD$174). (28)
GLP-1 Agonists and Surgical Outcomes
Pre-Clinical Studies
Liu et al. conducted a preclinical study using a mouse model to evaluate the effects of semaglutide vs. insulin on bone and joint infection (BJI) risk and neutrophil function following intra-articular implantation. (29) Female mice were fed a high-fat diet to induce obesity and type 2 diabetes before being treated with semaglutide (10 nmol/kg) or insulin (0.5 U/kg) for up to 28 days. (29) The mice then had S. aureus or E. coli inoculated K-wire inserted through the intercondylar notch of the distal femur. BJI rates were significantly lower in the semaglutide group for both bacterial challenges (S. aureus: 30% vs. 55%, p < 0.001; E. coli: 25% vs. 50%, p < 0.001). (29) Overall, semaglutide-treated mice exhibited a 47% reduction in infection rates compared to insulin-treated mice and a 61% reduction compared to untreated diabetic controls (p < 0.001). (29) The semaglutide group also showed a 41% increase in killing S. aureus (p = 0.002) and a 38% increase against E. coli (p = 0.004). (29) Likewise, reactive oxygen species production increased by 22% (p = 0.017) and neutrophil migration by 30% (p = 0.009). (29) Semaglutide-treated mice also exhibited a 19% reduction in body weight (p < 0.001) and a 36% improvement in glucose tolerance (p < 0.001), whereas insulin therapy had no significant effect on these metabolic parameters. (29)
Clinical Studies
Two retrospective cohort studies evaluated the direct impacts of GLP-1 agonists on surgical outcomes. Magruder et al. evaluated semaglutide use in 7,051 patients undergoing total knee arthroplasty (TKA) compared to a control group of 34,524 patients. (30, 31) Semaglutide use was associated with decreased odds of sepsis (0.0% vs. 0.4%; OR: 0.23, p < 0.001), prosthetic joint infection (PJI; 2.1% vs. 3.0%; OR: 0.70, p < 0.001), and 90-day readmissions (7.0% vs. 9.4%; OR: 0.71, p < 0.001). (30) Semaglutide users also trended toward fewer revision surgeries within two years (4.0% vs. 4.5%; OR: 0.86, p = 0.02) and lower 90-day postoperative costs ($15,291.66 vs. $16,798.46, p = 0.012). (30) However, they had higher odds of myocardial infarction (1.0% vs. 0.7%; OR: 1.49, p = 0.003), acute kidney injury (4.9% vs. 3.9%; OR: 1.28, p < 0.001), pneumonia (2.8% vs. 1.7%; OR: 1.67, p < 0.001), and hypoglycemic events (1.9% vs. 1.2%; OR: 1.55, p < 0.001). (30)
Magruder et al. also investigated the impact of semaglutide on postoperative outcomes in 9,465 patients with type 2 diabetes undergoing total hip arthroplasty (THA). (31) Patients prescribed semaglutide preoperatively (n = 1,653) were compared to a matched control group (n = 7,812) using a national claims database. (31) Semaglutide use significantly reduced 90-day readmission rates (6.2% vs. 8.8%; OR: 0.68, p < 0.01) and 2-year prosthetic joint infection rates (1.6% vs. 2.9%; OR: 0.56, p < 0.01). (31) The semaglutide group also demonstrated lower rates of revision surgery within two years (1.8% vs. 2.8%; OR: 0.64, p = 0.03), acute kidney injury (AKI) within 90 days (2.8% vs. 3.9%; OR: 0.69, p = 0.02), and hypoglycemic events (0.0% vs. 1.0%; OR: 0.45, p = 0.03). (31) Average 90-day global episode costs were lower for semaglutide users, trending toward significance ($13,219.92 vs. $14,681.71; p=0.06). (31) However, no significant differences were observed in other medical complications, implant-related complications, hospital length of stay, or same-day surgical costs. (31)
Discussion
The findings from this literature review suggest that GLP-1 receptor agonists influence bone metabolism, wound healing, and surgical outcomes, with both preclinical and clinical evidence supporting potential benefits in orthopedic settings. Their expanding role beyond weight management and glycemic control presents new considerations for bone remodeling, perioperative risk reduction, and postoperative recovery in orthopedic surgery. Given that more than half of total knee and hip arthroplasty patients meet criteria for obesity or morbid obesity, weight loss strategies, particularly pharmacologic approaches like GLP-1 receptor agonists, are becoming increasingly relevant in optimizing surgical outcomes and mitigating complications. However, their effects on bone integrity remain complex, with some evidence indicating bone-protective mechanisms while others suggest potential increases in bone resorption associated with weight loss.
The relationship between obesity and bone metabolism is paradoxical, as increased mechanical loading is traditionally associated with higher bone mineral density, yet adiposity-driven chronic inflammation and metabolic dysfunction impair bone quality. (6) Increased bone marrow adipose tissue accumulation and altered mesenchymal stem cell differentiation contribute to reduced osteoblast activity, increased osteoclastic resorption, and an overall decline in bone strength, leading to a greater fracture risk despite higher bone mineral density measurements. (12, 13) This review examined the extent to which GLP-1 receptor agonists modulate this metabolic environment, with preclinical and clinical studies presenting varying results.
Preclinical studies demonstrated that GLP-1 receptor agonists increased bone formation and reduced bone resorption in metabolic bone disease models, including diabetic osteoporosis, postmenopausal osteoporosis, and glucocorticoid-induced osteoporosis. (15-19) Liraglutide was found to improve bone microarchitecture and restore bone mass in rats with diabetic osteoporosis, as evidenced by upregulation of Wnt/β-catenin signaling and suppression of RANKL expression, leading to increased OPG and osteocalcin levels. (15-19) Abo-Elenin et al. demonstrated that semaglutide had similar effects in an ovariectomized rat model, increasing β-catenin expression and reducing RANKL production, which resulted in a net increase in bone formation. (17) Yang et al. found that liraglutide counteracted glucocorticoid-induced osteoporosis in a dexamethasone-treated rat model, with improvements in bone mineral density and increases in alkaline phosphatase and osteocalcin, both markers of osteoblast activity. (19) However, a study by Mieczkowska et al. found no improvements in bone strength or microarchitecture with liraglutide, suggesting that the effects of GLP-1 receptor agonists on bone remodeling may be context-dependent and influenced by metabolic conditions. (16)
Clinical studies provided conflicting evidence regarding the effects of GLP-1 receptor agonists on bone mineral density and bone metabolism markers. Iepsen et al. conducted a randomized controlled trial in which 37 obese women with a mean age of 46 years and a BMI of 34 kg/m² lost 12% of their body weight over 8 weeks and were then randomized to liraglutide or placebo for 52 weeks while maintaining their weight. (20) The liraglutide group preserved bone mineral content significantly better than the placebo group, with a 27g difference (95% CI: 5-48, p = .01). (20) Furthermore, the liraglutide group showed a 16% increase in P1NP, a bone formation marker, compared to a 2% increase in the placebo group (p < .05), while CTX-1, a bone resorption marker, remained unchanged in both groups. (20) These findings suggest that liraglutide may exert bone-protective effects by increasing bone formation. (20)
In contrast, Hansen et al. conducted a randomized controlled trial in a population with a mean age of 63 and increased fracture risk (T-score < -1.0 or a history of low-energy fractures). (21) They found that semaglutide was associated with increased CTX-1 levels (p = .021) and reduced BMD at weight-bearing sites, including the lumbar spine (p = .007), total hip (p = .001), and tibia (p = .003). (21) The observation by Hansen et al. that bone loss was most pronounced in weight-bearing skeletal sites, with no significant changes in non-weight-bearing areas such as the radius, suggests that mechanical unloading may be a key driver of bone resorption rather than a direct pharmacologic effect of GLP-1 receptor activation. (21) This aligns with the mechanostat theory, which posits that bone adapts to changes in mechanical forces by modulating turnover rates. (21) In the context of significant weight loss, reduced skeletal loading may lead to an imbalance favoring osteoclastic activity and bone resorption, which could explain the decreased BMD observed in semaglutide-treated patients. (21)
The discrepancy between the clinical trials by Iepson et al. and Hansen et al. may be attributable to differences in study populations, GLP-1 type, and the extent of weight loss achieved. (20, 21) Semaglutide was used with participants characterized as having an increased fracture risk due to a history of low-grade fracture and/or a T score of <-1.0, including both men and postmenopausal women. Additionally, the mean age of participants in the semaglutide study (63 years) was significantly higher than in the liraglutide study (46 years) and had a higher peak age of 85 compared to 65. The liraglutide study included only women, the majority of whom were not postmenopausal or with an increased fracture risk. Likewise, the goal of the liraglutide study was to maintain the pre-established weight loss to isolate the effects of GLP-1 agonists on bone metabolism. Further, the semaglutide study had 9 participants that needed to reduce their dose or stop entirely, as compared to the liraglutide group, in which all participants maintained the same dose.
GLP-1 receptor agonists were associated with reduced diabetic foot ulcer and lower limb amputation risk in retrospective cohort studies. Werkman et al. (2023) found that GLP-1 receptor agonists reduced the risk of lower limb amputation by 43% compared to sulfonylureas (HR: 0.57, 95% CI: 0.39–0.84), with the greatest risk reduction observed in long-term users (HR: 0.44, 95% CI: 0.27–0.72).(26) However, GLP-1 receptor agonists did not significantly impact diabetic foot ulcer incidence (HR: 1.08, 95% CI: 0.87–1.35). (26) In a follow-up study, Werkman et al. (2024) found that GLP-1 receptor agonists significantly reduced diabetic foot ulcer risk compared to insulin (HR: 0.44, 95% CI: 0.32–0.60) for short-term use and (HR: 0.74, 95% CI: 0.60–0.92) for long-term use. (27) These findings suggest potential benefits of GLP-1 receptor activation in vascular function, inflammation modulation, and tissue repair, which could have critical implications for post-surgical wound healing in orthopedic patients. (27)
This is contrasted with another study analyzing GLP-1 agonist effects on LLA, all-cause mortality, and healthcare costs. Chang et al. did not observe significant differences in amputation risk between GLP-1 receptor agonists, SGLT-2 inhibitors, and other diabetes medications in a large retrospective cohort study of 1,222,436 patients with type 2 diabetes. (28) The crude incidence rates of LLA were 6.1 per 10,000 person-years for GLP-1 receptor agonists, 1.5 for SGLT-2 inhibitors, 4.6 for DPP-4 inhibitors, and 1.9 for older agents. (28) However, GLP-1 agonists did have a lower all-cause mortality rate compared to other agents, but it came at the highest healthcare cost. (28)
The differences in findings between the Werkman et al. and Chang et al. studies may be attributed to several factors. (26-28) Study design and comparator groups varied, as Werkman et al. directly compared GLP-1 receptor agonists to insulin or sulfonylureas, whereas Chang et al. analyzed a broader range of diabetes medications without a direct reference group. (26-28) Additionally, patient populations differed, with Werkman et al. utilizing European datasets and Chang et al. using an Asian cohort, which may reflect differences in diabetes management, baseline cardiovascular risk, and genetic predisposition to amputation. (26-28) The duration of therapy and cumulative exposure to GLP-1 receptor agonists may also have played a role, as Werkman et al. found the most pronounced risk reduction in long-term users, whereas Chang et al. did not stratify results based on treatment duration. (26-28)
Preclinical and clinical studies suggest that GLP-1 receptor agonists may improve surgical outcomes by reducing infection rates and postoperative complications. Liu et al. conducted a murine study in which semaglutide-treated obese and diabetic mice had significantly lower rates of bone and joint infections compared to insulin-treated mice (S. aureus: 30% vs. 55%, p < 0.001; E. coli: 25% vs. 50%, p < 0.001). (29) Semaglutide treatment increased neutrophil bactericidal activity against S. aureus by 41% (p = .002) and against E. coli by 38% (p = .004), with improvements in superoxide production and neutrophil migration. (29) While this cannot be directly extrapolated to human outcomes, it does provide the beginnings of research that could be promising for reducing joint infections.
Magruder et al. conducted two retrospective cohort studies (2023, 2024) investigating the effects of semaglutide on perioperative outcomes in patients undergoing total knee arthroplasty (TKA) and total hip arthroplasty (THA). (30, 31) These studies analyzed large national claims databases to assess the impact of preoperative semaglutide use on postoperative complications, infection risk, and healthcare costs. (30,31) Both studies provided compelling evidence that semaglutide use is associated with reduced rates of prosthetic joint infections, sepsis, and hospital readmissions. However, they also reported an increased risk of certain medical complications, including myocardial infarction (MI) and acute kidney injury—findings that contradict earlier literature. (30,31) The authors acknowledged several limitations, including the use of a national patient database, which restricted their ability to control for prior diabetes management and preoperative glycemic control. (30) Additionally, the patient population differed from those in previous studies, as it excluded individuals with high cardiovascular risk. (30) The authors hypothesized that the observed increase in MI risk may be related to semaglutide’s action on the sinoatrial node and its potential to elevate heart rate. (30) As a result, selection bias may have influenced the outcomes.
The first study (2023) evaluated 7,051 patients undergoing TKA who were prescribed semaglutide preoperatively, comparing them to a matched control group of 34,524 non-users. (30) Semaglutide use was associated with significantly lower rates of prosthetic joint infections (2.1% vs. 3.0%; OR: 0.70, p < 0.001), sepsis (0.0% vs. 0.4%; OR: 0.23, p < 0.001), and 90-day readmissions (7.0% vs. 9.4%; OR: 0.71, p < 0.001). (30) Additionally, semaglutide use was linked to a trend toward lower revision surgery rates within two years (4.0% vs. 4.5%; OR: 0.86, p = 0.02) and reduced 90-day postoperative costs ($15,291.66 vs. $16,798.46, p = 0.012). (30) These findings suggest that semaglutide may play a role in reducing surgical-site infections and improving recovery outcomes by addressing key metabolic risk factors such as obesity, hyperglycemia, and systemic inflammation. (30)
However, semaglutide-treated patients also exhibited higher odds of myocardial infarction (1.0% vs. 0.7%; OR: 1.49, p = 0.003), acute kidney injury (4.9% vs. 3.9%; OR: 1.28, p < 0.001), pneumonia (2.8% vs. 1.7%; OR: 1.67, p < 0.001), and hypoglycemic events (1.9% vs. 1.2%; OR: 1.55, p < 0.001). (30) The mechanism underlying these increased risks remains unclear, though they may be related to semaglutide’s effects on cardiovascular function, fluid balance, and insulin-glucagon dynamics. (30) Rapid weight loss and volume shifts may contribute to perioperative hemodynamic instability, particularly in older or higher-risk patients. (30) While weight loss and metabolic improvements generally confer cardioprotective benefits, rapid weight reduction may introduce transient risks in certain high-risk surgical populations. (30)
In a second study, Magruder et al. (2024) examined 9,465 patients with type 2 diabetes undergoing THA, comparing 1,653 semaglutide users to a matched control group of 7,812 non-users. (31) Similar to the TKA study, semaglutide use was associated with reduced 90-day readmission rates (6.2% vs. 8.8%; OR: 0.68, p < 0.01), lower two-year prosthetic joint infection rates (1.6% vs. 2.9%; OR: 0.56, p < 0.01), and fewer revision surgeries within two years (1.8% vs. 2.8%; OR: 0.64, p = 0.03). (31) Additionally, semaglutide users had lower rates of acute kidney injury within 90 days (2.8% vs. 3.9%; OR: 0.69, p = 0.02) and hypoglycemic events (0.0% vs. 1.0%; OR: 0.45, p = 0.03). (31) Unlike the TKA study, no significant differences were observed in other perioperative complications such as venous thromboembolism, pneumonia, or cardiovascular events. (31) This disparity may be attributed to confounding variables between patient populations, such as THA patients exhibiting better baseline cardiovascular health compared to other cohorts.
Taken together, the Magruder et al. studies provide compelling evidence that semaglutide use in orthopedic surgery is associated with lower rates of infection, readmission, and revision surgery, likely due to improved metabolic control, reductions in systemic inflammation, and weight loss-driven perioperative risk reduction. (30, 31) However, they also highlight potential cardiovascular and renal risks, emphasizing the need for careful perioperative monitoring and patient selection. Future studies should explore whether perioperative hydration protocols, electrolyte monitoring, or cardiovascular screening could help mitigate these risks while maximizing the benefits of GLP-1 agonists in orthopedic surgical populations.
Some orthopedic surgeons may perceive the growing use of GLP-1 receptor agonists as a potential threat to their practice, fearing that widespread weight loss could reduce the volume of patients requiring joint replacements. However, this perspective overlooks the broader impact of these medications on optimizing surgical outcomes, reducing complications, and expanding access to surgery for higher-risk patients. While obesity is a primary driver of osteoarthritis and the need for joint arthroplasty, it also contributes to increased perioperative morbidity, higher infection rates, longer hospital stays, and worse long-term functional outcomes. Many obese patients are denied surgery or experience poor recovery due to metabolic dysfunction, delayed wound healing, and elevated cardiopulmonary risk. By incorporating GLP-1 receptor agonists into preoperative optimization strategies, surgeons can expand their eligible surgical population, improve patient selection, and enhance long-term implant survival and patient satisfaction. Rather than reducing demand for orthopedic procedures, these medications may facilitate better patient outcomes, fewer complications, and a shift toward higher-quality, lower-risk surgical care, ultimately benefiting both patients and orthopedic surgeons.
While this review focused primarily on adult populations, there is limited research available on the use of GLP-1 therapies in pediatric patients. A review by Alorfi et al. examined 19 clinical trials that investigated the use of exenatide, semaglutide, and liraglutide as pharmacologic options for treating childhood obesity alongside behavioral and lifestyle interventions, as well as surgical approaches. (33) However, only 8 of these trials met the inclusion criteria for analysis. (33) The limited data underscore the need for further research to clarify appropriate dosing, treatment duration, and the potential effects on growth and development in pediatric populations. (33) Notably, this review did not report any data on the impact of GLP-1 therapies on musculoskeletal health in children.
Additionally, data on the use of GLP-1 therapies in geriatric populations remain limited. A review by Karagiannis et al. examined 11 studies involving patients over the age of 65 receiving GLP-1 receptor agonist treatments. (34) However, the focus of this review was primarily on the cardiovascular and renal benefits of these therapies, with little to no emphasis on musculoskeletal outcomes. (34) Given the increased risk of sarcopenia, osteoporosis, and frailty in older adults, future research should explore the long-term effects of GLP-1 receptor agonists on musculoskeletal health in this population, alongside their established systemic benefits.
There are several limitations to this literature review. The studies varied in design, patient population, and outcome measures, making direct comparisons challenging. Many of the clinical studies relied on retrospective analyses, which are subject to selection bias and unmeasured confounders. Additionally, preclinical animal models provide mechanistic insights but may not fully translate to human physiology, particularly in conditions such as osteoporosis, obesity-related bone changes, and joint infections. Furthermore, long-term fracture risk, bone microarchitecture changes, and implant survival in GLP-1 agonist users remain poorly studied. Prospective, longitudinal studies are needed to further elucidate these effects and optimize perioperative strategies in orthopedic populations. Additionally, future research should explore whether adjunctive therapies, such as resistance training or bone-specific pharmacologic interventions, could counteract potential bone resorption associated with weight loss or be superior to GLP-1s.
As GLP-1 receptor agonists continue to be widely adopted for obesity and metabolic disease, it is essential to consider their broader implications for bone health and orthopedic surgery. The existing literature suggests that while these agents may help optimize metabolic and surgical outcomes, their effects on bone remain highly context-dependent, influenced by factors such as age, baseline bone health, duration of therapy, and degree of weight loss. A recent review by Gatto et al., published in May 2025, reached a similar conclusion—suggesting that GLP-1 receptor agonists may have a positive impact on musculoskeletal health. (35) Proposed benefits include anti-inflammatory effects, modulation of bone metabolism, and preservation of skeletal muscle during weight loss. However, the authors likewise emphasize the need for further research to better understand the long-term musculoskeletal effects of these agents. (35)
Conclusion
GLP-1 receptor agonists are becoming integral to obesity and diabetes management, with significant implications for orthopedic surgery. Their ability to improve metabolic health, reduce systemic inflammation, and lower surgical complication rates makes them valuable in optimizing patient outcomes. Preclinical and clinical studies suggest potential effects on bone metabolism, with evidence that GLP-1 receptor activation may support bone formation and remodeling. However, the relationship between GLP-1 therapy, weight loss, and bone health remains complex and context-dependent. Orthopedic surgeons must weigh their benefits and potential therapeutic use in improving metabolic and perioperative outcomes. Clinical studies are scarce, highlighting the need for further research to assess bone quality and long-term skeletal outcomes in individuals using GLP-1s.
References
- National Institute of Diabetes and Digestive and Kidney Diseases. Overweight & Obesity Statistics. National Institutes of Health. Updated May 2023. https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity
- Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;(96):156-161. doi:10.1136/postgradmedj-2019-137186
- Jain S. 2023 Trends Shaping the Health Economy Report. Trilliant Health; 2023. https://www.trillianthealth.com/hubfs/2023%20Trends%20Shaping%20the%20Health%20Economy%20Report.pdf
- American Diabetes Association Professional Practice Committee. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S52-S76. doi:10.2337/dc24-S004
- Watanabe JH, Kwon J, Nan B, Reikes A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J Am Pharm Assoc JAPhA. 2024;64(1):133-138. doi:10.1016/j.japh.2023.10.002
- Welton KL, Gagnier JJ, Urquhart AG. Proportion of Obese Patients Presenting to Orthopedic Total Joint Arthroplasty Clinics. Orthopedics. 2016;39(1):e127-e133. doi:10.3928/01477447-20151222-12
- Carender CN, Glass NA, DeMik DE, Elkins JM, Brown TS, Bedard NA. Projected Prevalence of Obesity in Primary Total Knee Arthroplasty: How Big Will the Problem Get? J Arthroplasty. 2022;37(7):1289-1295. doi:10.1016/j.arth.2022.03.003
- Kulkarni K, Karssiens T, Kumar V, Pandit H. Obesity and osteoarthritis. Maturitas. 2016;89:22-28. doi:10.1016/j.maturitas.2016.04.006
- Moscatelli F, Monda A, Messina G, et al. Exploring the Interplay between Bone Marrow Stem Cells and Obesity. Int J Mol Sci. 2024;25(5):2715. doi:10.3390/ijms25052715
- Rinonapoli G, Pace V, Ruggiero C, et al. Obesity and Bone: A Complex Relationship. Int J Mol Sci. 2021;22(24):13662. doi:10.3390/ijms222413662
- Zhou L, Jang KY, Moon YJ, et al. Leptin ameliorates ischemic necrosis of the femoral head in rats with obesity induced by a high-fat diet. Sci Rep. 2015;5(1):9397. doi:10.1038/srep09397
- Onggo JR, Onggo JD, de Steiger R, Hau R. Greater risks of complications, infections, and revisions in the obese versus non-obese total hip arthroplasty population of 2,190,824 patients: a meta-analysis and systematic review. Osteoarthritis Cartilage. 2020;28(1):31-44. doi:10.1016/j.joca.2019.10.005
- Luo G, Liu H, Lu H. Glucagon-like peptide-1(GLP-1) receptor agonists: potential to reduce fracture risk in diabetic patients? Br J Clin Pharmacol. 2016;81(1):78-88. doi:10.1111/bcp.12777
- Simental-Mendía LE, Sánchez-García A, Linden-Torres E, Simental-Mendía M. Effect of glucagon-like peptide-1 receptor agonists on circulating levels of leptin and resistin: A meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2021;177:108899. doi:10.1016/j.diabres.2021.108899
- Fathy MA, Anbaig A, Aljafil R, et al. Effect of Liraglutide on Osteoporosis in a Rat Model of Type 2 Diabetes Mellitus: A Histological, Immunohistochemical, and Biochemical Study. Microsc Microanal Off J Microsc Soc Am Microbeam Anal Soc Microsc Soc Can. 2023;29(6):2053-2067. doi:10.1093/micmic/ozad102
- Mieczkowska A, Millar P, Chappard D, Gault VA, Mabilleau G. Dapagliflozin and Liraglutide Therapies Rapidly Enhanced Bone Material Properties and Matrix Biomechanics at Bone Formation Site in a Type 2 Diabetic Mouse Model. Calcif Tissue Int. 2020;107(3):281-293. doi:10.1007/s00223-020-00720-4
- Abo-Elenin MHH, Kamel R, Nofal S, Ahmed AAE. The crucial role of beta-catenin in the osteoprotective effect of semaglutide in an ovariectomized rat model of osteoporosis. Naunyn Schmiedebergs Arch Pharmacol. 2025;398(3):2677-2693. doi:10.1007/s00210-024-03378-z
- Pal S, Maurya SK, Chattopadhyay S, et al. The osteogenic effect of liraglutide involves enhanced mitochondrial biogenesis in osteoblasts. Biochem Pharmacol. 2019;164:34-44. doi:10.1016/j.bcp.2019.03.024
- Yang L, Yang J, Pan T, Zhong X. Liraglutide increases bone formation and inhibits bone resorption in rats with glucocorticoid-induced osteoporosis. J Endocrinol Invest. 2019;42(9):1125-1131. doi:10.1007/s40618-019-01034-5
- Iepsen EW, Lundgren JR, Hartmann B, et al. GLP-1 Receptor Agonist Treatment Increases Bone Formation and Prevents Bone Loss in Weight-Reduced Obese Women. J Clin Endocrinol Metab. 2015;100(8):2909-2917. doi:10.1210/jc.2015-1176
- Hansen MS, Wölfel EM, Jeromdesella S, et al. Once-weekly semaglutide versus placebo in adults with increased fracture risk: a randomised, double-blinded, two-centre, phase 2 trial. EClinicalMedicine. 2024;72:102624. doi:10.1016/j.eclinm.2024.102624
- Palermo A, D’Onofrio L, Eastell R, Schwartz AV, Pozzilli P, Napoli N. Oral anti-diabetic drugs and fracture risk, cut to the bone: safe or dangerous? A narrative review. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2015;26(8):2073-2089. doi:10.1007/s00198-015-3123-0
- Wikarek A, Grabarczyk M, Klimek K, Janoska-Gawrońska A, Suchodolska M, Holecki M. Effect of Drugs Used in Pharmacotherapy of Type 2 Diabetes on Bone Density and Risk of Bone Fractures. Med Kaunas Lith. 2024;60(3):393. doi:10.3390/medicina60030393
- Antonopoulou M, Bahtiyar G, Banerji MA, Sacerdote AS. Diabetes and bone health. Maturitas. 2013;76(3):253-259. doi:10.1016/j.maturitas.2013.04.004
- Herrou J, Mabilleau G, Lecerf JM, Thomas T, Biver E, Paccou J. Narrative Review of Effects of Glucagon-Like Peptide-1 Receptor Agonists on Bone Health in People Living with Obesity. Calcif Tissue Int. 2024;114(2):86-97. doi:10.1007/s00223-023-01150-8
- Werkman NCC, Driessen JHM, Klungel OH, et al. Incretin-based therapy and the risk of diabetic foot ulcers and related events. Diabetes Obes Metab. 2024;26(9):3764-3780. doi:10.1111/dom.15721
- Werkman NCC, Driessen JHM, Stehouwer CDA, et al. The use of sodium-glucose co-transporter-2 inhibitors or glucagon-like peptide-1 receptor agonists versus sulfonylureas and the risk of lower limb amputations: a nation-wide cohort study. Cardiovasc Diabetol. 2023;22(1):160. doi:10.1186/s12933-023-01897-2
- Chang HY, Chou YY, Tang W, et al. Association of antidiabetic therapies with lower extremity amputation, mortality and healthcare cost from a nationwide retrospective cohort study in Taiwan. Sci Rep. 2021;11(1):7000. doi:10.1038/s41598-021-86516-4
- Liu T, Zhou L, Chen Y, Lin J, Zhu H. Semaglutide outperforms insulin in restoring neutrophil function against implant-related infection in diabetic and obese mice: experimental research. Int J Surg. 2025;111(1):273-282. doi:10.1097/JS9.0000000000001896
- Magruder ML, Yao VJH, Rodriguez AN, Ng MK, Sasson V, Erez O. Does Semaglutide Use Decrease Complications and Costs Following Total Knee Arthroplasty? J Arthroplasty. 2023;38(11):2311-2315.e1. doi:10.1016/j.arth.2023.05.071
- Magruder ML, Miskiewicz MJ, Rodriguez AN, Mont MA. Semaglutide Use Prior to Total Hip Arthroplasty Results in Fewer Postoperative Prosthetic Joint Infections and Readmissions. J Arthroplasty. 2024;39(3):716-720. doi:10.1016/j.arth.2023.12.023
- Jiao Y, Sun J, Li Y, Zhao J, Shen J. Association between Adiposity and Bone Mineral Density in Adults: Insights from a National Survey Analysis. Nutrients. 2023;15(15):3492. doi:10.3390/nu15153492
- Alorfi NM, Alshehri FS. Usage of Glucagon-Like Peptide-1 for Obesity in Children; Updated Review of Clinicaltrials.gov. J Multidiscip Healthc. 2023;16:2179-2187. Published 2023 Jul 31. doi:10.2147/JMDH.S419245
- Karagiannis T, Tsapas A, Athanasiadou E, et al. GLP-1 receptor agonists and SGLT2 inhibitors for older people with type 2 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;174:108737. doi:10.1016/j.diabres.2021.108737
- Gatto A, Liu K, Milan N, Wong S. The Effects of GLP-1 Agonists on Musculoskeletal Health and Orthopedic Care. Curr Rev Musculoskelet Med. Published online May 15, 2025. doi:10.1007/s12178-025-09978-3


