Himanshu Rao, DO1; Christopher Ferguson, DO1; Shaan Sadhwani, DO1; Brian Omslaer, DO1; Daniel Kim, DO2
1Department of Orthopaedic Surgery, UPMC Community Osteopathic, Harrisburg, PA, USA
2Orthopedic Institute of Pennsylvania, Harrisburg, PA, USA
DOI: 10.70709/bfik4ioe4z
Abstract
Case
A patient that underwent dual mobility liner placement presented at follow up with clinical history and imaging findings indicative of metallosis. This phenomenon with dual mobility implants is not well reported in the literature.
Conclusion
Although dual mobility implants allow for increased stability of the acetabular component, the benefits conferred should be weighed against the potential complications. Ensuring proper cup placement and adequate screw fixation can potentially mitigate the risk of metallosis in these cases.
Keywords: Total joint arthroplasty, dual mobility, metallosis, hip
Introduction
The dual mobility (DM) implant, initially introduced by G. Bousquet in the 1970s, has become a key innovation in total hip arthroplasty (THA), particularly in high dislocation risk patients 1. By increasing the femoral head-neck ratio and employing a metal acetabular cup with an additional metal liner, the design enhanced stability and significantly reduced the risk of dislocation throughout a wider range of motion2. It gained widespread acceptance, especially among patients with spinal fusions or recurrent hip instability issues3 4 However, with the growing use of dual mobility implants, new complications have emerged, notably metallosis, a condition associated with metal-on-metal implants.
Metallosis is the dark, stained appearance of the joint capsule and surrounding tissues due to the accumulation of metallic debris, formed from components in metal on metal articulations2. Metallic ions are ingested at the cellular level causing an adverse local tissue response (ALTR). The inflammatory response that is elicited can lead to tissue deterioration with formation of pseudotumors, aseptic lymphocyte dominated vasculitis associated lesions (ALVAL), and trunnionosis in addition to metallosis. Diagnosis is often complicated by nonspecific symptoms that present similarly to prosthetic joint infection, instability or aseptic loosening 5. Trunionosis, another complication of modular implants during which the neck of the femoral component interacts inappropriately to impinge on other metal components is another closely related complication6. One case series of trunnion corrosion in metal-on-polyethylene THA, mean preoperative serum cobalt was 7.1 ppb (range 2.2–12.8) and mean chromium was 2.2 ppb (range 0.5–5.2). Another series involving recalled cobalt-chromium femoral heads reported mean serum cobalt of 8.4 ± 7.0 μg/L and chromium of 3.4 ± 3.3 μg/L, with a mean cobalt/chromium ratio of 3.7. Although both studies were grossly within what was quantified as the normal reference range for these concentrations, they are significantly elevated compared to average levels in THA patients without trunnionosis or metal related complications.7,8. However, recent literature has revealed that serum ion levels may be a reliable tool for assisting in diagnosis with serum cobalt greater than 1 ng/mL and cobalt/chromium ratio above 2 suggestive of disease 9–11. If elevated levels are detected and patient is sympotmatic for signs of metallosis, surgical removal of hardware should be considered first line treatment. In the setting of a nonsurgical candidate, attempt can be made at chelation therapy, with N-acetylcysteine although this is not standard of care and described in limited case reports12. However, high systemic metallic ion concentrations may not directly correlate with patient symptoms and severity of disease 13. Additionally, MRI and CT imaging can help determine the extent.13 Early screening and detection has the potential to prevent morbid complications and economic burden related to metallosis12.
While extensively studied in traditional metal-on-metal systems, the incidence of metallosis in dual mobility THAs remains poorly understood. The incidence of metallosis in all THAs is reportedly 5.3%, but MoM bearings have a 2-3 fold higher rate of metal related revisions for MoM implants 12,13. Our investigation of the literature only found two studies that documented metallosis in the setting of a dual mobility implant. The first study was as a result of dislodging of the polyethylene bearing, causing the metal femoral head component to erode against the metal liner14. The second study we found, was a prospective analysis of dislocations following total hip arthroplasty. Periarticular metallosis with was reported in 69% of these patients (105 of 153). With visible wear related lesions on the dual mobility cup metal shell and/or femoral neck were in 65% of these patients15. Our report aims to detail a case of this rare phenomenon in a patient with a dual mobility THA with the goal of examining the pathophysiology of metallosis in dual mobility implants and reviewing the existing literature on the topic.
Case History
Figure 1: Initial radiographs depicting developmental dysplasia of the left hip and the index anterior total hip arthroplasty performed.
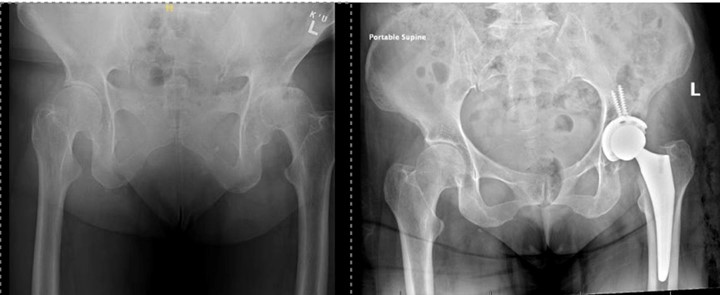
Figure 2: Revision L THA with dual mobility component.
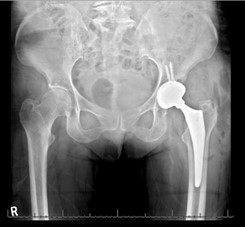
Figure 3: (a) Initial R aTHA (b) 1-year follow up depicting acetabular loosening and vertical cup migration.
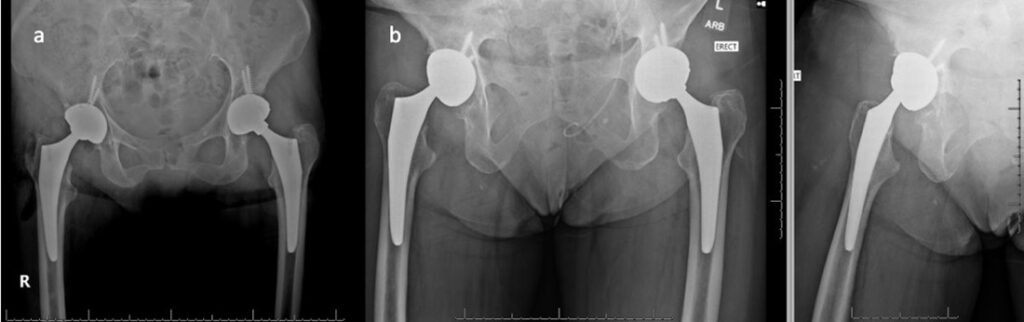
Figure 4: CT and MRI of right hip demonstrating pseudotumor formation
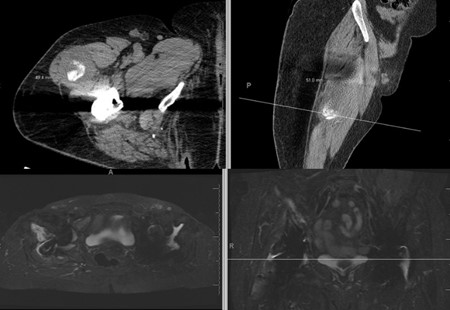
Figure 5: Intraoperative findings showing pseudotumor formation and metallosis
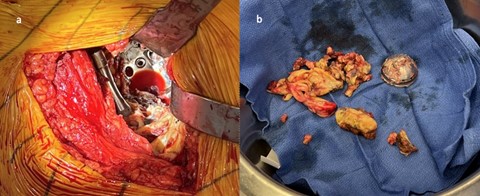
Figure 6: Initial post operative radiographs after metallosis revision demonstrating adequate positioning and screw placement
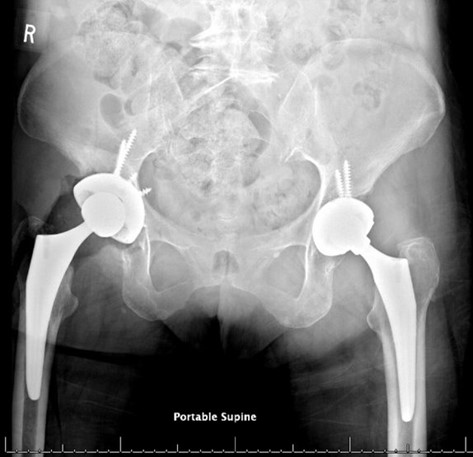
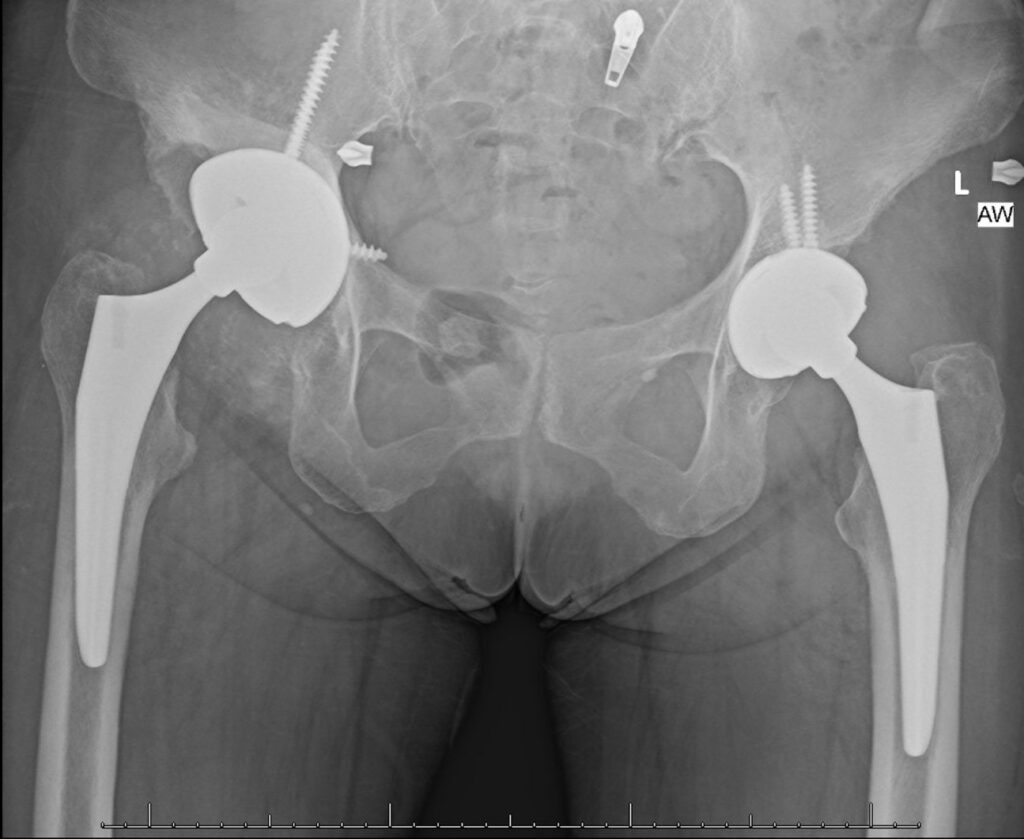
Figure 7: Second revision radiographs with revision of screw, cup and neck
The patient was informed that the details about the case would be submitted for publication, and written permission was obtained. A 70-year-old female with history of developmental dysplasia of the left hip underwent direct anterior total hip arthroplasty (aTHA) (Figure 1). The post-operative course was complicated by two anterior dislocations 2 months after her index procedure, and after the second dislocation, patient underwent implantation of a dual mobility (DM) component (Figure 2). After an uncomplicated postoperative course, the patient underwent right dual mobility aTHA placement 10 months later given multiple contralateral dislocations (Figure 3). The patient then presented for a 1-year follow up reporting a 4-months of worsening groin pain necessitating cane use. Radiographs demonstrated loosening of the acetabular component as evidenced by increased inclination angle comparted to initial postoperative films (Figure 3). At this point, further radiographic and lab workup was obtained. Laboratory workup revealed a C-reactive protein (CRP) of 3.3 mg/dL and erythrocyte sedimentation rate of 22 mm/hr. A nuclear medicine bone scan showed abnormal activity in multiple areas of the right iliac bone and computed-tomography (CT) scan showed a fracture extending into the iliac wing with callous formation and a mass-like region of heterogenous attenuation extending from the superior acetabulum to the proximal femur (Figure 4a). Magnetic resonance imaging (MRI) revealed a large 11.6cm x 5.5cm x 13.8cm rim enhancing fluid collection inferior to her THA. (Figure 4b). Hip aspiration was performed and brown, cloudy fluid with a total nucleated cell count of 35,750 with 36% neutrophils and 54% lymphocytes was obtained. All cultures from this aspiration were negative. Other labs obtained included cobalt and chromium levels which were 8.6 (ng/mL) and 2.2 (ng/mL) respectively. Alpha-defensin lateral flow test was also obtained and found to be negative.
Based on laboratory findings, negative cultures, and imaging studies demonstrating component lossening, ALTR, and metallosis were suspected rather than infectious etiology. The patient was scheduled for revision surgery. The prior anterior incision was used and a pseudotumor was encountered (Figure 5a). Upon resection and debridement, it was found that while the femoral component was stable, the DM liner was loose. The screw heads did not appear prominent on initial evaluation. Acetabular component screws and cup were then removed (Figure 5b). The acetabulum was then reamed to 63 mm and cancellous bone chips along with demineralized bone matrix was impacted into the contained defects. A 64 mm cup (Zimmer, Warsaw, IN) was then implanted and fixed with 2 posterior superior screws (Zimmer, Warsaw, IN) in the posterior column, 2 screws in the anterior superior portion of the column, and 1 screw in the ischium. With regard to the iliac wing fracture, we decided to manage non-operatively and trend her exam.
Post-operatively the patient was made flat foot weight bearing. She was ambulating pain-free at her 12 week follow up appointment. However, the initial revision set the stage for chronic instability secondary to the tissue damage and bone loss caused by the metallosis. Within 6 months of her revision, she experienced three dislocations of her right hip, requiring a second revision procedure with a larger cup while continuing the use of a dual mobility implant to preserve stability. Our report will focus on her initial surgery which led to her metallosis and her first revision procedure.
Discussion
Metallosis is a well-recognized complication of metal-on-metal (MoM) and modular component implants, resulting from wear debris generated at articulating and non-articulating surfaces. Released metallic ions provoke an inflammatory response, leading to tissue necrosis and pseudotumor formation. While traditionally associated with MoM bearings, the phenomenon has also been reported in non-MoM designs due to corrosion or micromotion at modular interfaces 13,16.
Dual mobility implants are predominantly used for increased stability in patients at high-risk for dislocation. The dual mobility design features a polyethylene bearing which articulates with a cobalt chrome metal liner that is fixed inside of a hemispherical multi bearing acetabular shell. It has been theorized that micromotion between this metal liner and the acetabular shell has the potential to cause fretting corrosion and subsequent release of metal ions 17. However, reports of metallosis and pseudotumor formation associated with dual mobility constructs are limited in their comprehensiveness, and there has been no definitive mechanism to which metallosis in these cases can be attributed.
It is important to recognize proper surgical technique and any modifiable factors that could decrease the risk of micromotion and metal debris when using a DM construct.
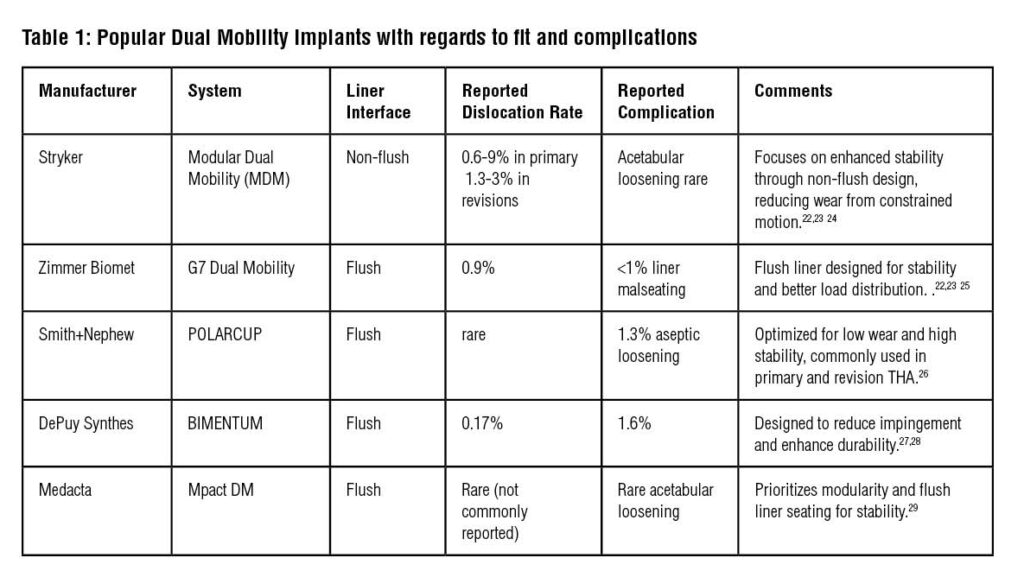
It is also crucial to understand the design features of the dual mobility implant used to understand how to optimally seat the implant interfaces to avoid potential complications (Table 1). The following represent key design features of dual mobility systems and implant placement may influence the risk of complications:
- Coaxial Liner Seating: Improper alignment of the cobalt chrome metal liner within the acetabular shell can lead to edge loading and micromotion, contributing to early implant failure. Ensuring precise, coaxial seating during implantation is critical 18. Not all liners are manufactured to sit flush with the acetabular shell (Table 1, Figure 8)
- Cup Fixation Techniques: The use of screws for acetabular fixation is common in cases with deficient bone stock. However, as illustrated in this case, screw toggling during aseptic loosening can exacerbate wear at the metal interface. Exploring alternative fixation methods or refining screw designs may help mitigate this issue19,20. If using screws, it is important to fully seat them as any screw head prominence may prevent proper fixation of the metal liner inside the acetabular shell.
- Surface Coatings and Materials: Advanced coatings, such as hydroxyapatite or porous titanium, are designed to enhance osseointegration and reduce micromotion. These features may be particularly beneficial in dual mobility implants where micromotion at multiple interfaces poses a risk21.

We hypothesize that in our case, aseptic loosening around the screws in the acetabular shell led to toggling and loosening of the shell which exacerbated micromotion between the DM cobalt chrome liner and the acetabular shell. This created a cycle of increasing metal ion release, ALTR, metallosis and increased loosening. In this patient, although screws were well-seated intraoperatively, subsequent toggling forces at the screw-cup interface led to wear and contributing to metal debris. While the dual mobility construct provided increased stability, the resultant extensive metallosis and pseudotumor formation, combined with elevated cobalt and chromium levels, underscore the risks associated with micromotion in this implant.
Our case highlights that while screws may improve initial stability, their role in subsequent wear-related complications warrants further consideration. We acknowledge that our patient’s clinical course has been turbulent and fraught with further complications from dislocations and a second revision procedure which highlights how devastating metallosis can be for future stability. Close postoperative monitoring, including early imaging and serum metal ion evaluation in symptomatic patients, is critical for early detection and management of similar complications. Furthermore, while this study is aimed at highlighting an otherwise under-reported complication, larger and more comprehensive studies are warranted to examine the phenomenon of metallosis secondary to dual-mobility acetabular component utilization.
Conclusion
Dual mobility implants have demonstrated significant advantages in reducing dislocation rates, particularly in high-risk populations such as patients with dysplasia or recurrent instability. However, this case emphasizes the potential for complications, including metallosis and pseudotumor formation, when fixation or positioning is suboptimal.
References
- Charnley J. The long-term results of low-friction arthroplasty of the hip performed as a primary intervention. J Bone Joint Surg Br. 1972;54(1):61-76.
- De Martino I, Triantafyllopoulos GK, Sculco PK, Sculco TP. Dual mobility cups in total hip arthroplasty. World J Orthop. 2014;5(3):180-187. doi:10.5312/wjo.v5.i3.180
- Murphy EP, Fenelon C, Russell S, Condon F. Cause of irreducible dislocation of a re-revision THR. BMJ Case Rep. 2018;2018:bcr2017223072, bcr-2017-223072. doi:10.1136/bcr-2017-223072
- Pai FY, Ma HH, Chou TFA, et al. Risk factors and modes of failure in the modern dual mobility implant. A systematic review and meta-analysis. BMC Musculoskelet Disord. 2021;22(1):541. doi:10.1186/s12891-021-04404-4
- Della Valle CJ, Calkins TE, Jacobs JJ. Diagnosing Taper Corrosion: When Is It the Taper and When Is It Something Else? J Arthroplasty. 2018;33(9):2712-2715. doi:10.1016/j.arth.2018.01.054
- Marinier M, Edmiston TA, Kearns S, Hannon CP, Levine BR. A Survey of the Prevalence of and Techniques to Prevent Trunnionosis. Orthopedics. 2018;41(4):e557-e562. doi:10.3928/01477447-20180524-03
- Lachiewicz PF, O’Dell JA. Trunnion corrosion in metal-on-polyethylene hip arthroplasty: a simple diagnosis and treatment? Bone Jt J. 2018;100-B(7):898-902. doi:10.1302/0301-620X.100B7.BJJ-2017-1127.R2
- Urish KL, Hamlin BR, Plakseychuk AY, et al. Trunnion Failure of the Recalled Low Friction Ion Treatment Cobalt Chromium Alloy Femoral Head. J Arthroplasty. 2017;32(9):2857-2863. doi:10.1016/j.arth.2017.03.075
- Plummer DR, Berger RA, Paprosky WG, Sporer SM, Jacobs JJ, Della Valle CJ. Diagnosis and Management of Adverse Local Tissue Reactions Secondary to Corrosion at the Head-Neck Junction in Patients With Metal on Polyethylene Bearings. J Arthroplasty. 2016;31(1):264-268. doi:10.1016/j.arth.2015.07.039
- Fillingham YA, Della Valle CJ, Bohl DD, et al. Serum Metal Levels for Diagnosis of Adverse Local Tissue Reactions Secondary to Corrosion in Metal-on-Polyethylene Total Hip Arthroplasty. J Arthroplasty. 2017;32(9S):S272-S277. doi:10.1016/j.arth.2017.04.016
- Hall DJ, Pourzal R, Jacobs JJ. What Surgeons Need to Know About Adverse Local Tissue Reaction in Total Hip Arthroplasty. J Arthroplasty. 2020;35(6S):S55-S59. doi:10.1016/j.arth.2020.01.016
- Gessner BD, Steck T, Woelber E, Tower SS. A Systematic Review of Systemic Cobaltism After Wear or Corrosion of Chrome-Cobalt Hip Implants. J Patient Saf. 2019;15(2):97-104. doi:10.1097/PTS.0000000000000220
- Ude CC, Esdaille CJ, Ogueri KS, et al. The Mechanism of Metallosis After Total Hip Arthroplasty. Regen Eng Transl Med. 2021;7(3):247-261. doi:10.1007/s40883-021-00222-1
- Mohammed R, Cnudde P. Severe metallosis owing to intraprosthetic dislocation in a failed dual-mobility cup primary total hip arthroplasty. J Arthroplasty. 2012;27(3):493.e1-3. doi:10.1016/j.arth.2010.11.019
- Wegrzyn J, Malatray M, Pibarot V, Anania G, Béjui-Hugues J. Is Isolated Mobile Component Exchange an Option in the Management of Intraprosthetic Dislocation of a Dual Mobility Cup? Clin Orthop. 2020;478(2):279-287. doi:10.1097/CORR.0000000000001055
- Oliveira CA, Candelária IS, Oliveira PB, Figueiredo A, Caseiro-Alves F. Metallosis: A diagnosis not only in patients with metal-on-metal prostheses. Eur J Radiol Open. 2015;2:3-6. doi:10.1016/j.ejro.2014.11.001
- Matsen Ko LJ, Pollag KE, Yoo JY, Sharkey PF. Serum Metal Ion Levels Following Total Hip Arthroplasty With Modular Dual Mobility Components. J Arthroplasty. 2016;31(1):186-189. doi:10.1016/j.arth.2015.07.035
- Guyen O, Pibarot V, Vaz G, Chevillotte C, Béjui-Hugues J. Use of a dual mobility socket to manage total hip arthroplasty instability. Clin Orthop. 2009;467(2):465-472. doi:10.1007/s11999-008-0476-0
- Lee A, Paiement GD, Penenberg BL, Rajaee SS. Metallosis in Total Hip Arthroplasty. JBJS Rev. 2023;11(10). doi:10.2106/JBJS.RVW.23.00105
- Rahman TM, Frisch NB, Darrith B, Patel I, Silverton CD. Incidence of Pseudotumors in a Dual Modular Stem Construct With and Without Metal-on-Metal Bearing Surface. J Am Acad Orthop Surg. 2021;29(2):e92-e97. doi:10.5435/JAAOS-D-19-00652
- Bohara S, Suthakorn J. Surface coating of orthopedic implant to enhance the osseointegration and reduction of bacterial colonization: a review. Biomater Res. 2022;26(1):26. doi:10.1186/s40824-022-00269-3
- Guntin J, Plummer D, Valle CD, DeBenedetti A, Nam D. Malseating of modular dual mobility liners. Bone Jt Open. 2021;2(10):858-864. doi:10.1302/2633-1462.210.BJO-2021-0124.R1
- Siljander MP, Gausden EB, Wooster BM, et al. Liner malseating is rare with two modular dual-mobility designs. Bone Jt J. 2022;104-B(5):598-603. doi:10.1302/0301-620X.104B5.BJJ-2021-1734.R1
- Modular Dual Mobility. Accessed June 1, 2025. https://www.stryker.com/us/en/joint-replacement/products/modular-dual-mobility.html
- 2336.4-GLBL-en G7 Acetabular System Surgical Technique-digital1.pdf. Accessed June 1, 2025. https://www.zimmerbiomet.com/content/dam/zb-corporate/en/education-resources/surgical-techniques/specialties/hip/g7-acetabular-system/2336.4-GLBL-en%20G7%20Acetabular%20System%20Surgical%20Technique-digital1.pdf
- Bauchu P, Bonnard O, Cyprès A, Fiquet A, Girardin P, Noyer D. The dual-mobility POLARCUP: first results from a multicenter study. Orthopedics. 2008;31(12 Suppl 2):orthosupersite.com/view.asp?rID=37180.
- Lustig S, Cotte M, Foissey C, Asirvatham RD, Servien E, Batailler C. Monobloc dual-mobility acetabular component versus a standard single-mobility acetabular component. Bone Jt J. 2024;106-B(3 Supple A):81-88. doi:10.1302/0301-620X.106B3.BJJ-2023-0572.R1
- BI-MENTUMTM Dual Mobility System | DePuy Synthes. J&J MedTech. Accessed June 1, 2025. https://www.jnjmedtech.com/en-US/product/bi-mentum-dual-mobility-system
- Medacta Corporate. Accessed June 1, 2025. https://www.medacta.com/EN/mpact-system


